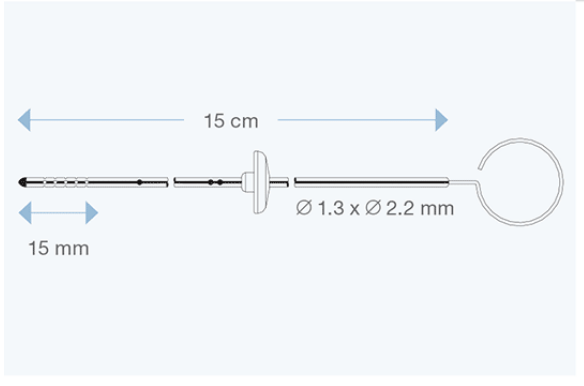
Available in different sizes for both children and adults, they are made of transparent silicone elastomer, with a radiopaque stripe entirely embedded within the silicone wall, and with a tantalum-labelled tip. All models are delivered with right angle adapter and introducing stylet.

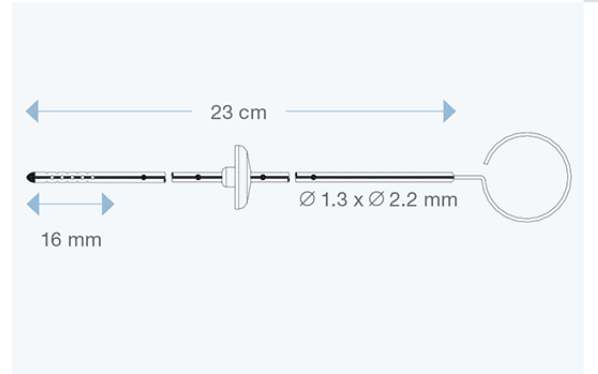
Available in different sizes for both children and adults, they are made of transparent silicone elastomer, with a radiopaque stripe entirely embedded within the silicone wall, and with a tantalum-labelled tip. All models are delivered with right angle adapter and introducing stylet.

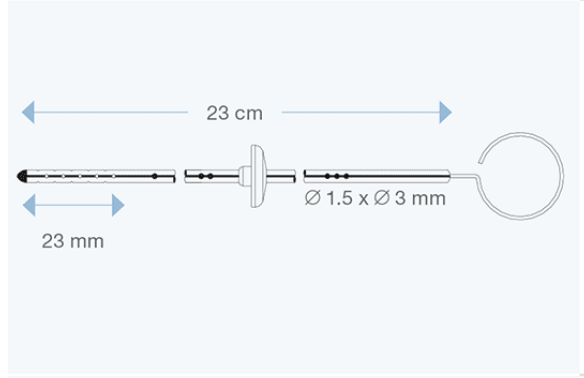
Available in different sizes for both children and adults, they are made of transparent silicone elastomer, with a radiopaque stripe entirely embedded within the silicone wall, and with a tantalum-labelled tip. All models are delivered with right angle adapter and introducing stylet.

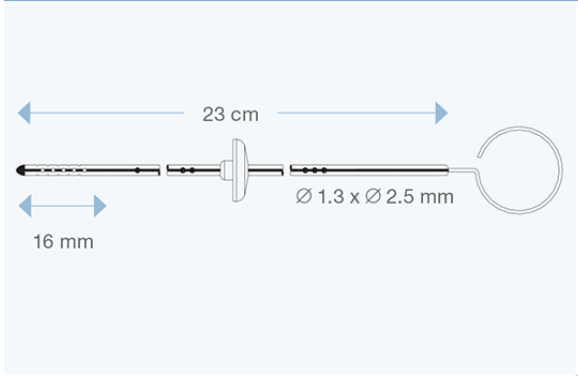
Available in different sizes for both children and adults, they are made of transparent silicone elastomer, with a radiopaque stripe entirely embedded within the silicone wall, and with a tantalum-labelled tip. All models are delivered with right angle adapter and introducing stylet.


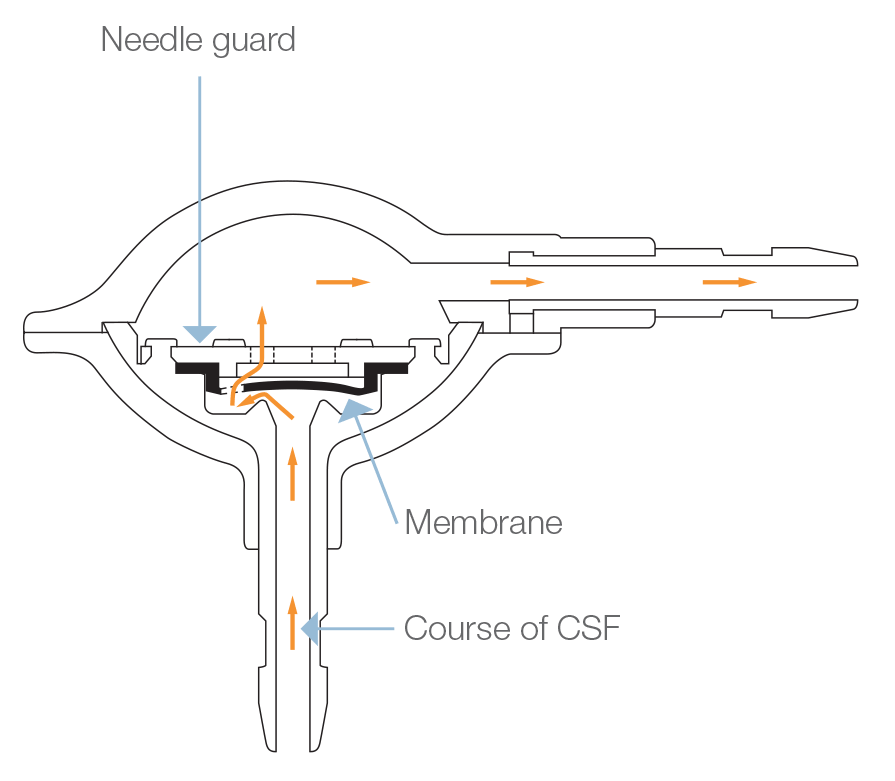
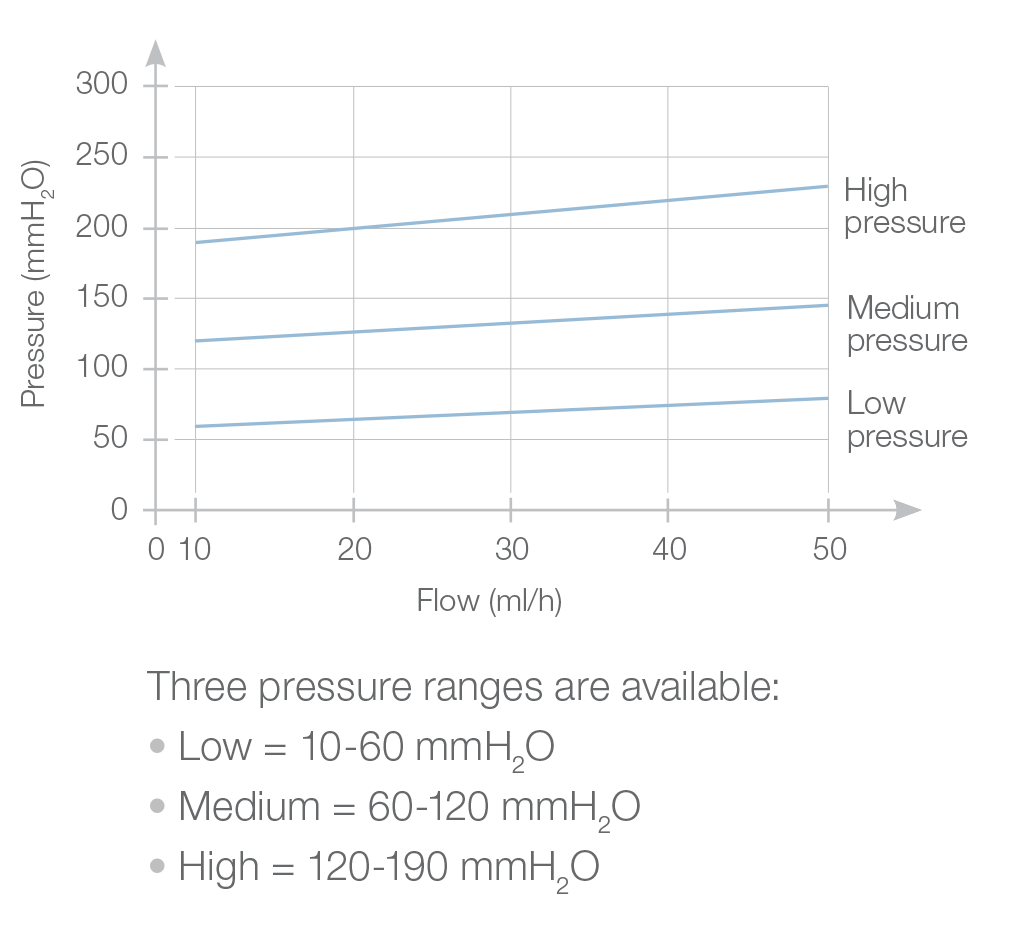
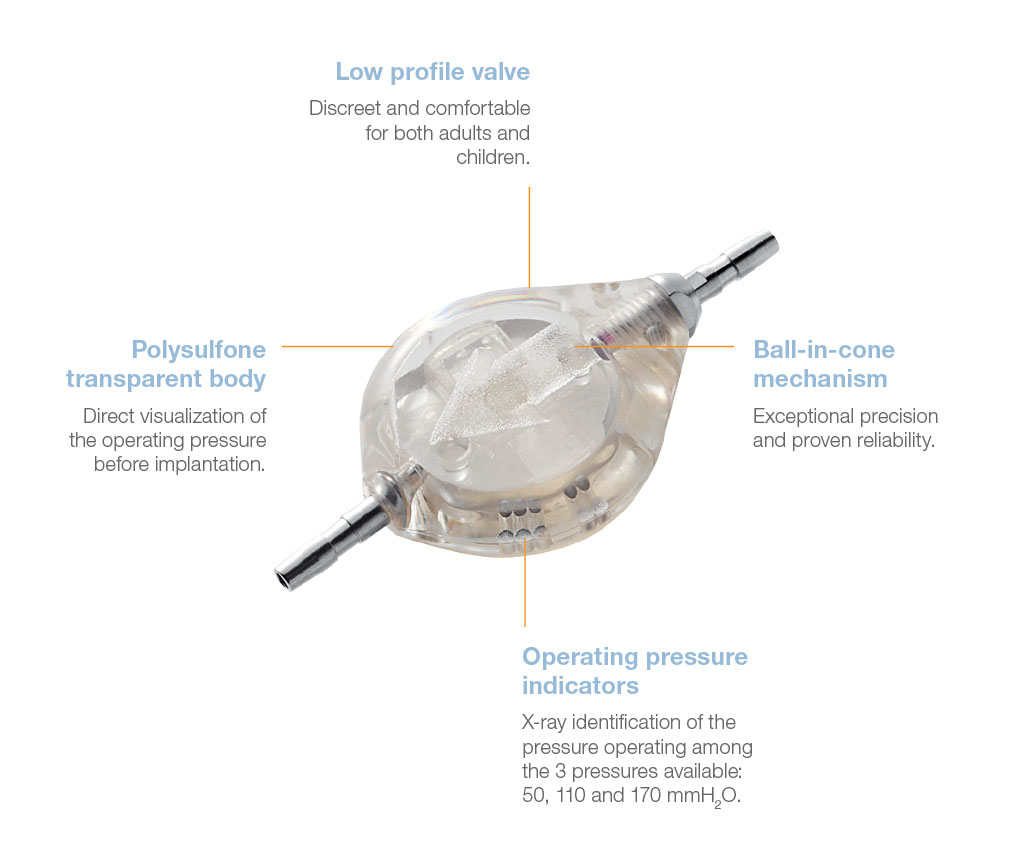
The Pulsar® valve is a monopressure valve for cerebrospinal fluid drainage. Its principle is based on the play of a silicone membrane, calibrated in low, medium or high pressure, ensuring a proximal regulation of the CSF flow through the shunt system.
The Pulsar® valve has an anti-reflux system, offers the possibility to test the shunt patency and gives access to CSF.
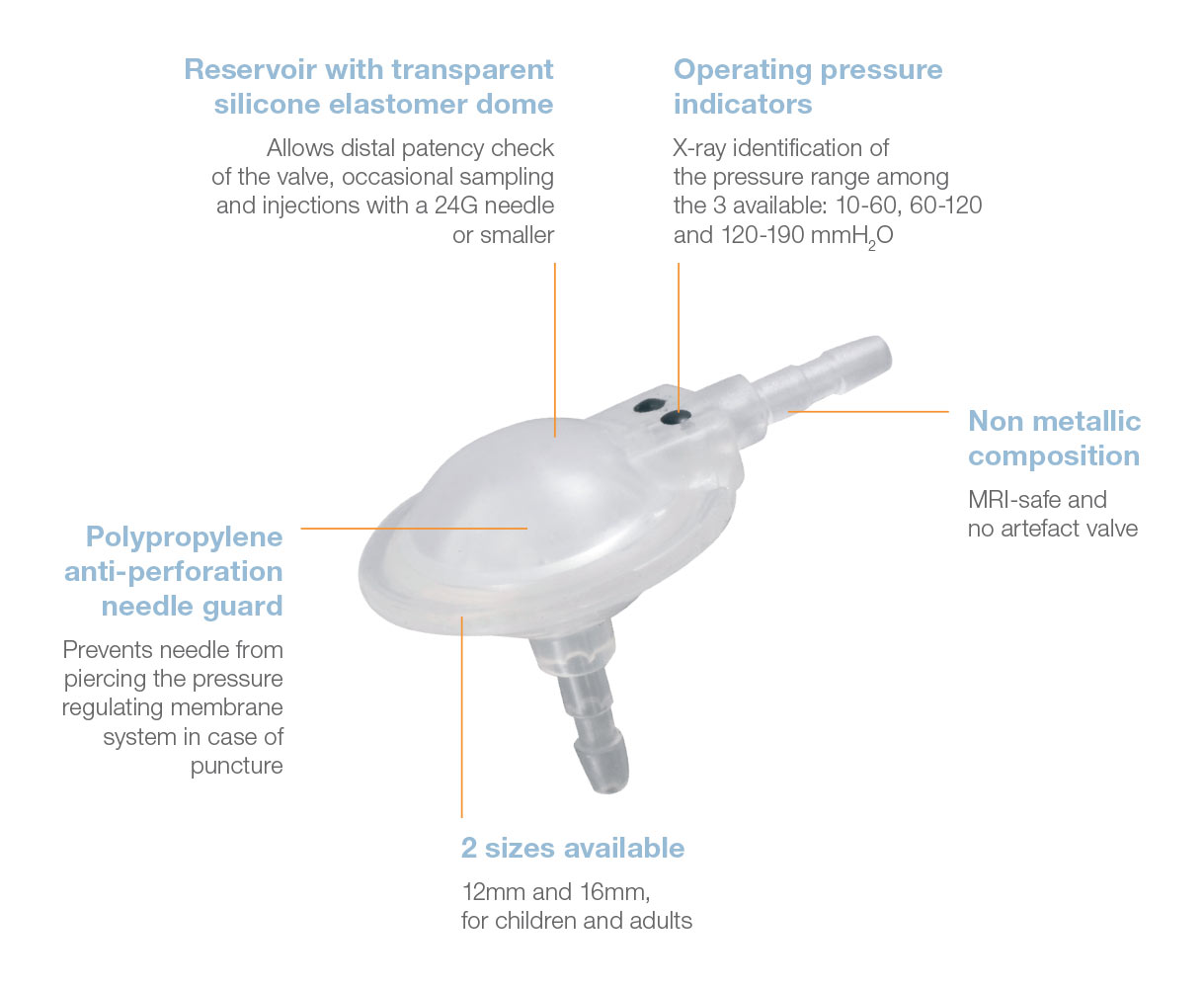
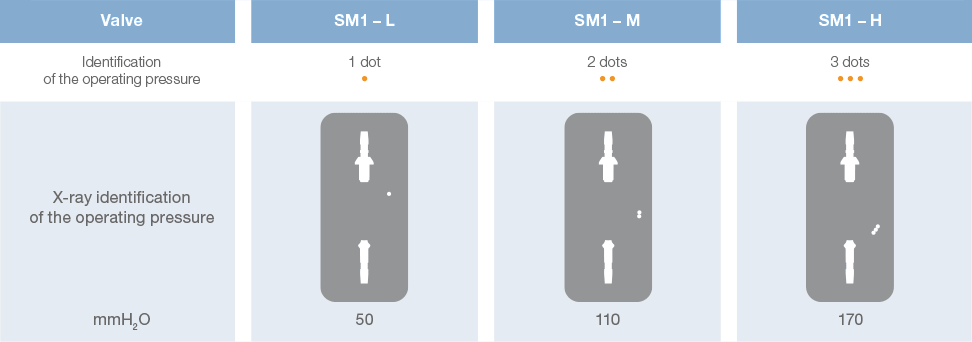
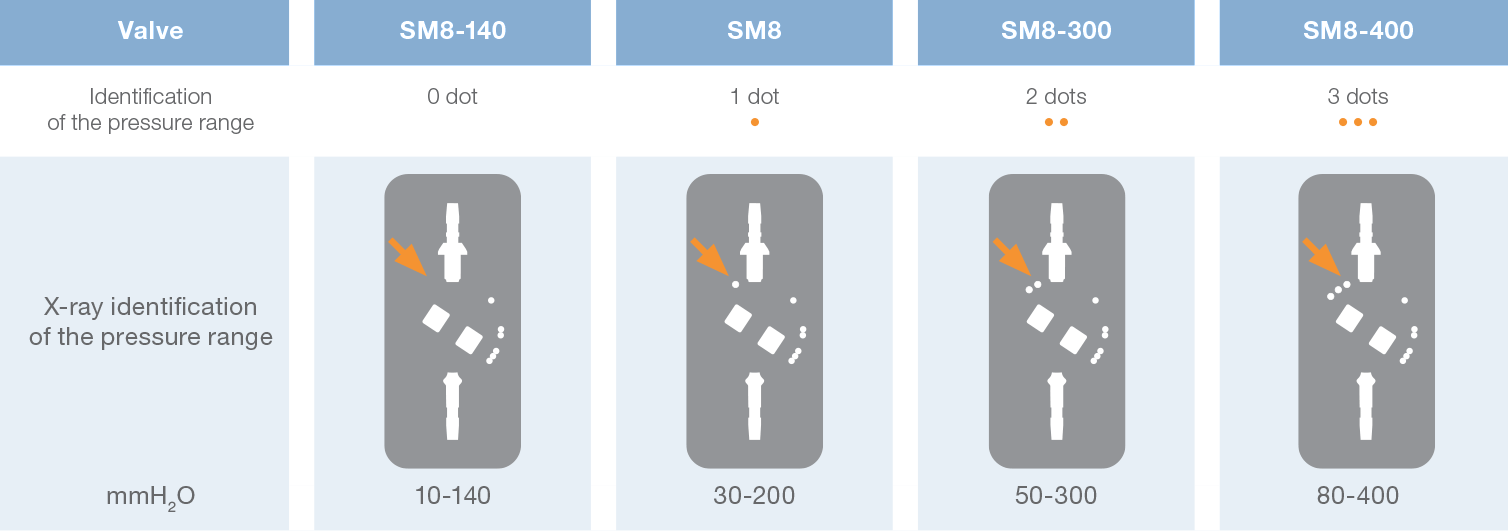
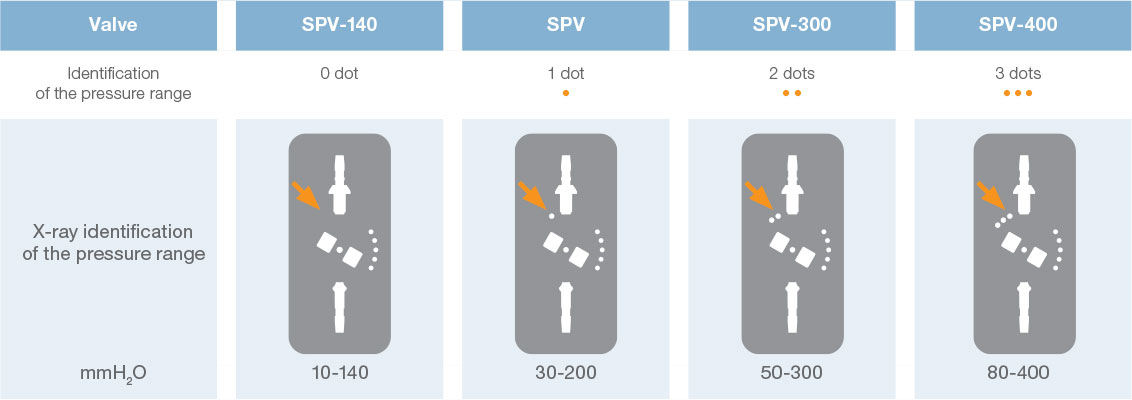
Direct x-ray identification of the operating pressure is possible thanks to radiopaque dots on the valve.
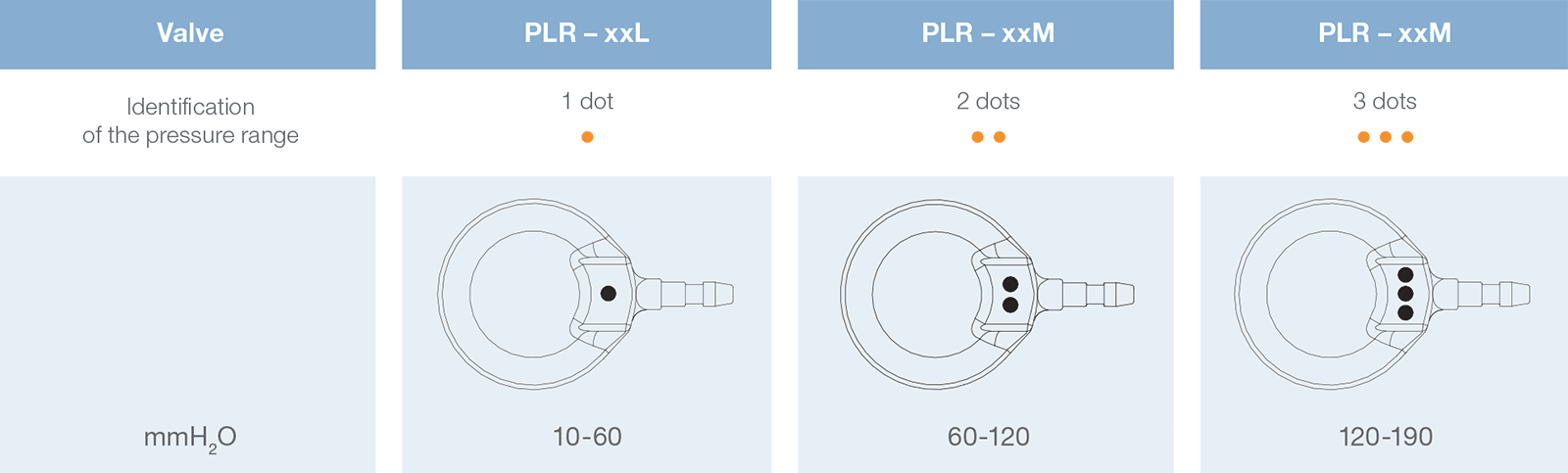
xx = 12 or 16 mm, depending on the size selected.

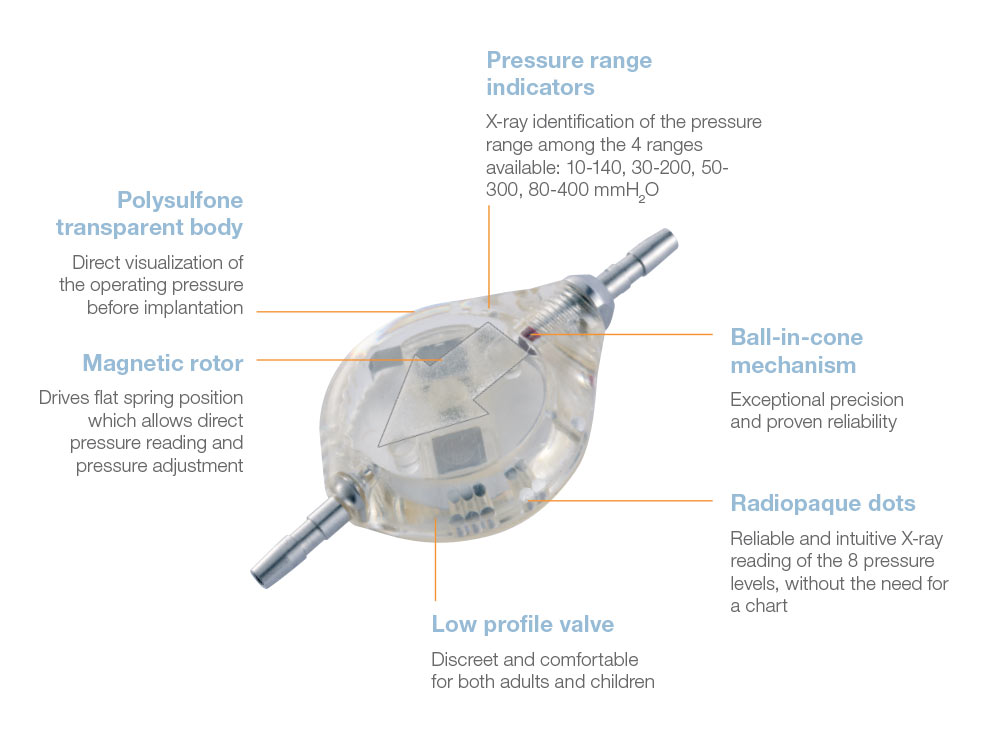
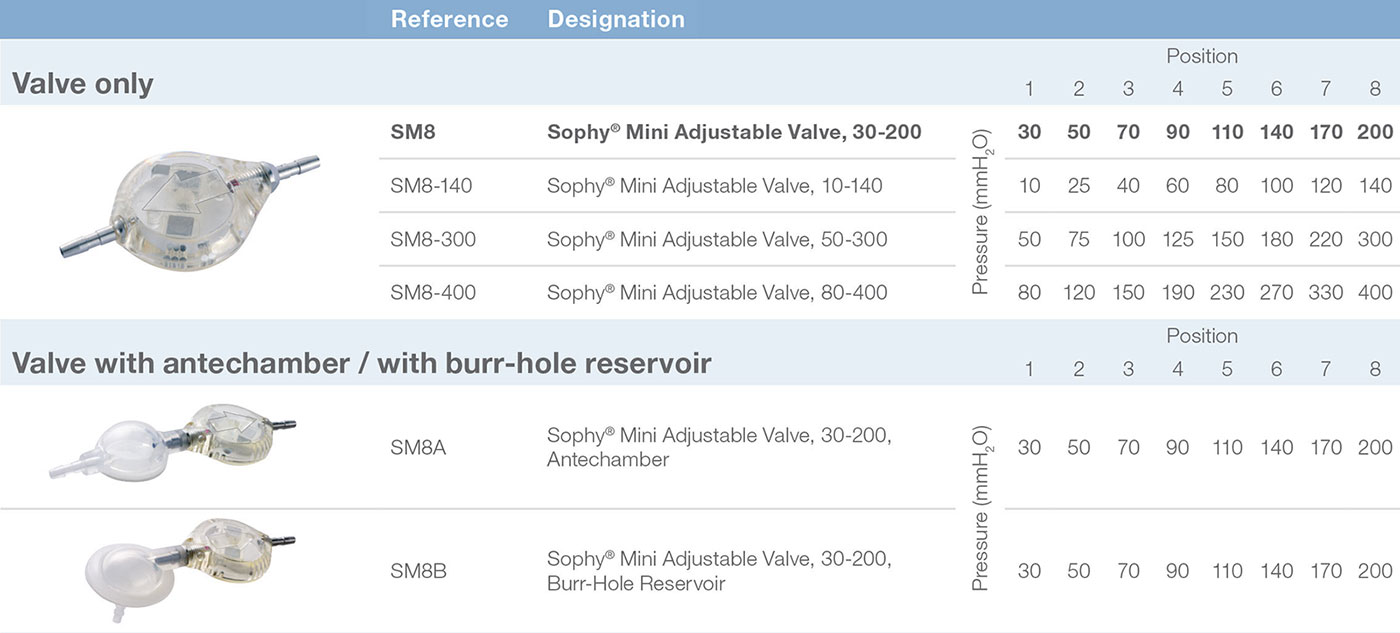
The Adjustable Sophy® Mini valve is a high-precision device of unparalleled simplicity.
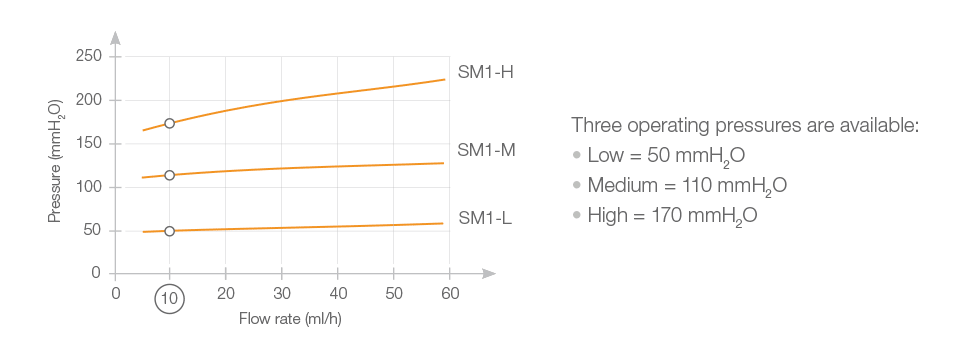
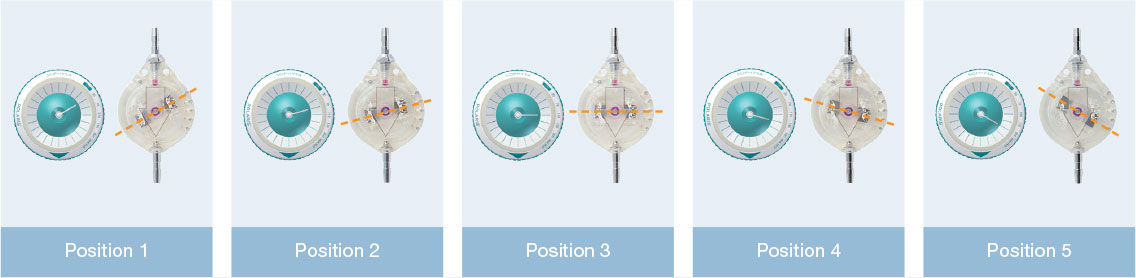
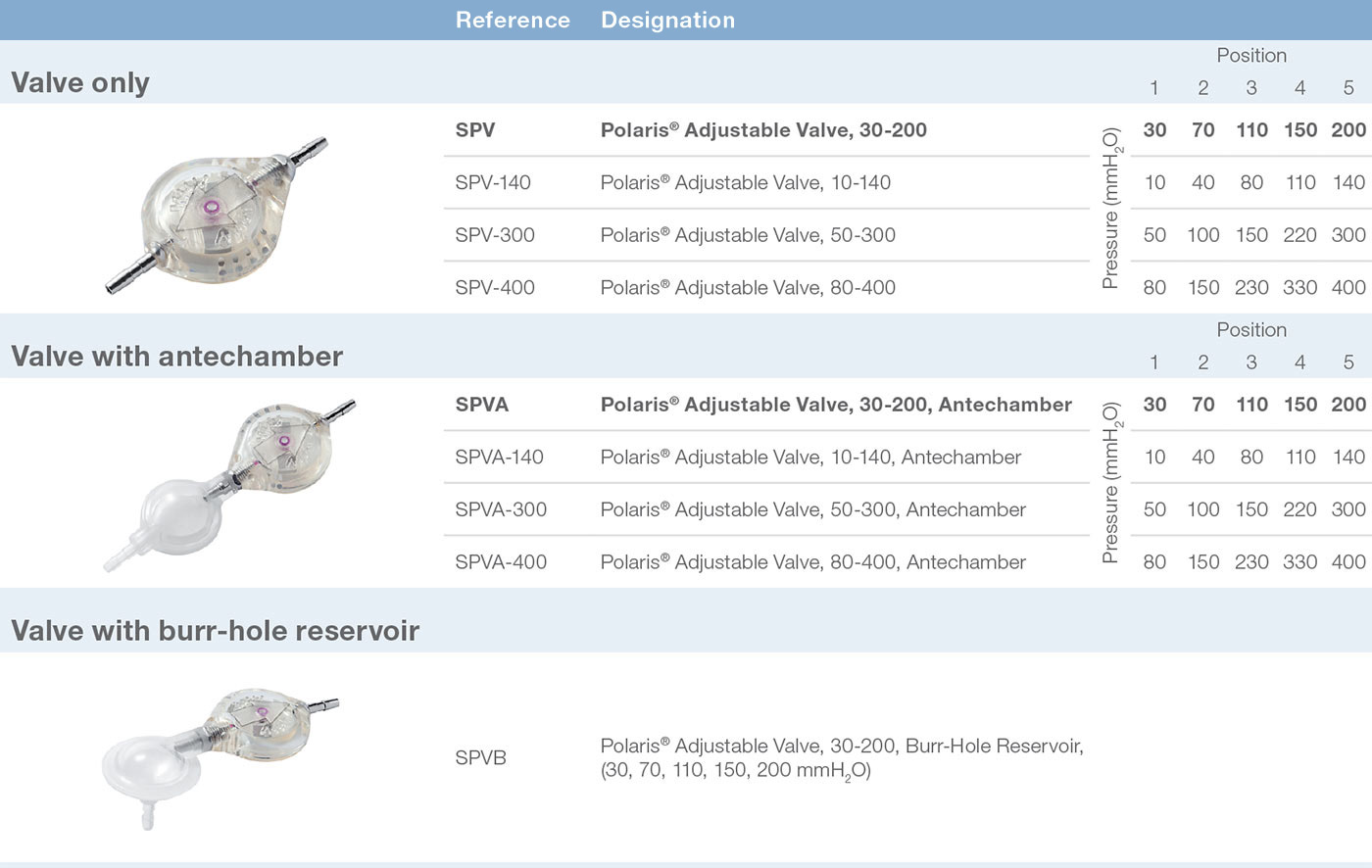
The principle of the Sophy® valve resides in the variation in pressure exerted on a ball by a semi-circular spring at various points along its circumference. The spring is attached to a magnetic rotor which position can be non-invasively changed using an adjustment magnet. A series of indentations allows a variety of positions to be selected, each position representing a different pressure setting.
The Sophy® Mini Monopressure valve (SM1) is a low-profile high precision ball-in-cone valve. It has the small size, the shape of the Sophysa Adjustable Valves and is made of the same material.
Direct x-ray identification of the operating pressure is possible thanks to radiopaque dots on the valve.
The Sophy® Mini SM1 can be associated with SiphonX®, an anti-siphon device, which adds 200 mmH 2O in vertical position.

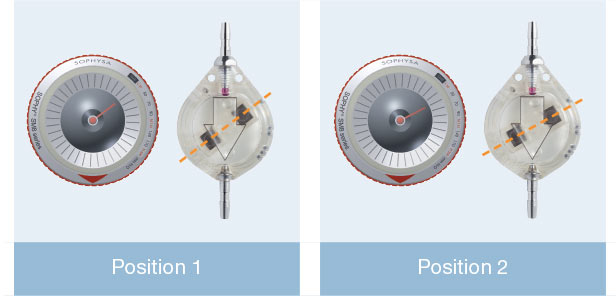
The Adjustable Sophy® Mini valve is a high-precision device of unparalleled simplicity.
The principle of the Sophy® valve resides in the variation in pressure exerted on a ball by a semi-circular spring at various points along its circumference. The spring is attached to a magnetic rotor which position can be non-invasively changed using an adjustment magnet. A series of indentations allows a variety of positions to be selected, each position representing a different pressure setting.
Direct pressure reading is obtained using the adjustment kit compass: the compass needle is aligned with the position of the magnetic rotor.
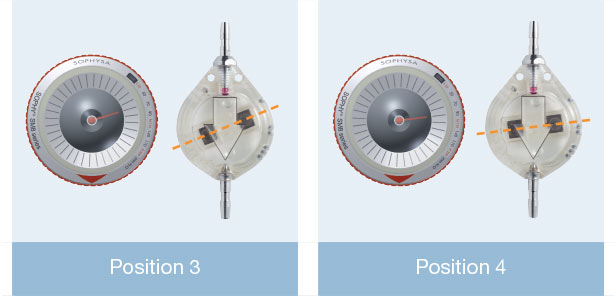


In addition to the standard model (30-200 mmH 2O), Sophy® Mini SM8 also offers a unique special pressure range: one low pressure valve and two high pressure valves. Thus a choice is provided, depending on the experience of the practitioner, to meet very special needs
The Sophy® Mini SM8-140 and SM8 can be associated with SiphonX®, an anti-siphon device, which adds 200 mmH 2O in vertical position.

The safety of adjustable valves has become a major concern for neurosurgeons because of the growing use of electromagnetic devices in daily life and the development of high power MRI (3 teslas).
Indeed, these devices are liable to modify the selected pressure accidentally, with the risk of disrupting CSF drainage and causing serious complications for the patient.
The Polaris® valve is a major breakthrough for the safety of patients fitted with adjustable valves.
Its exclusive locking mechanism enables it to resist:
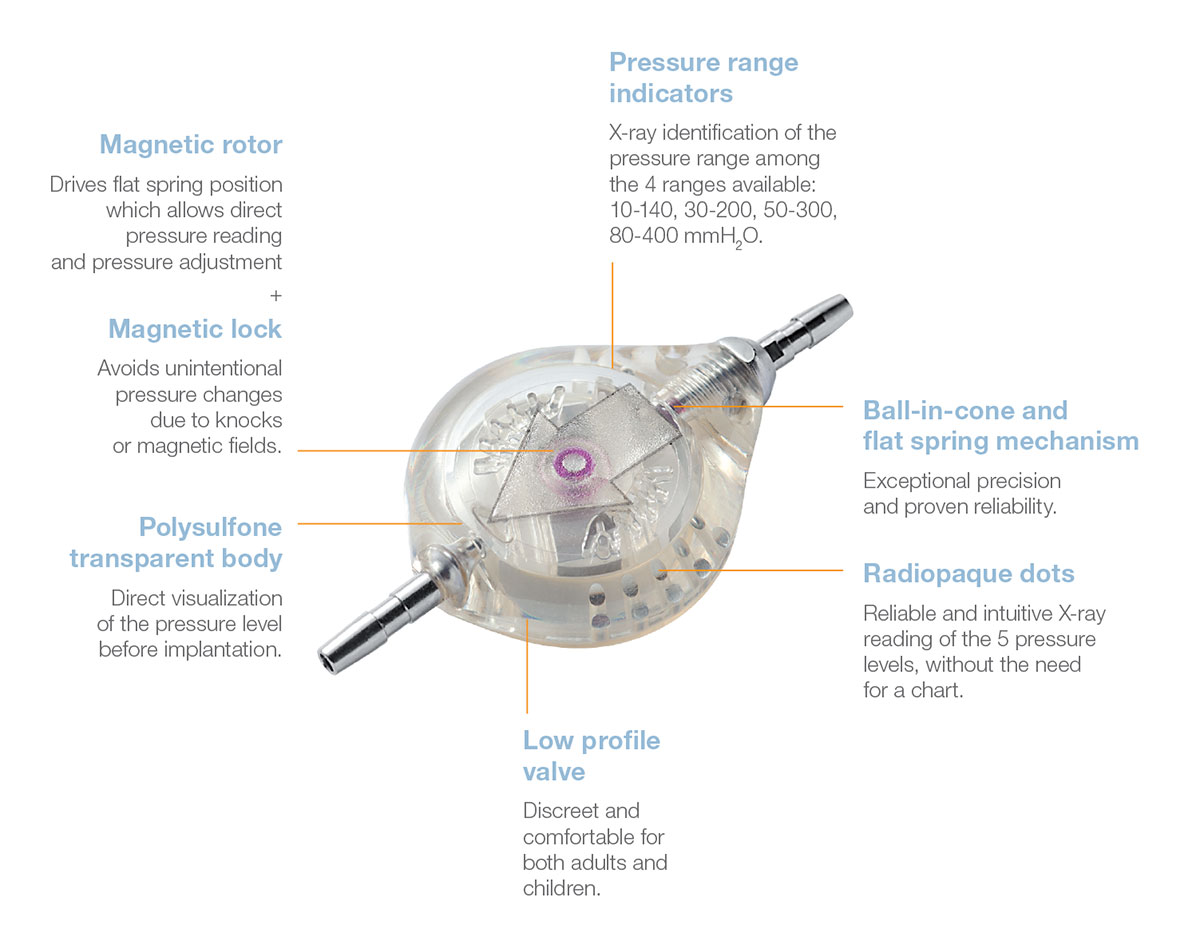
The adjustable Polaris® valve is a major breakthrough for the safety of patients thanks to the patented self-locking system of the rotor.
This magnetic lock has been designed to resist unintentional operating pressure changes due to knocks or exposure to magnetic fields, especially during MRI examinations.
It offers the patient an unequalled security against the clinical risks associated with those dysadjustments.
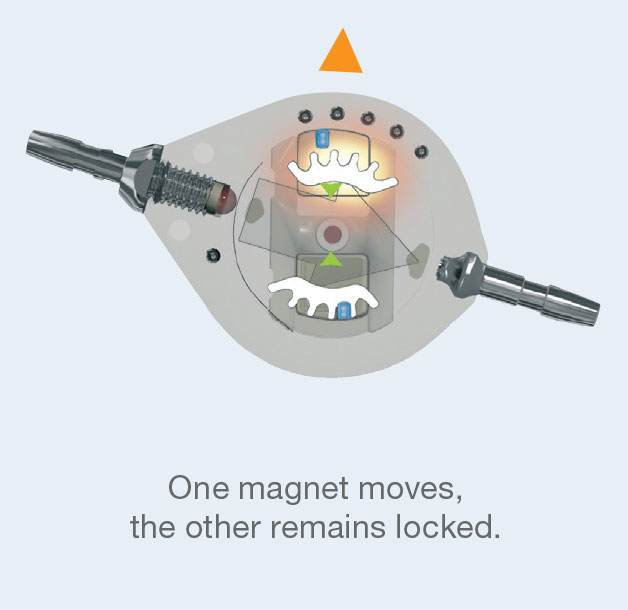
The Polaris® Magnetic Lock is based on the permanent reciprocal attraction of two mobile micro-magnets of opposite polarity.
This “magnetic lock" holds the rotor in the selected position, thus preventing any accidental change in operating pressure if the valve is exposed
to magnetic fields.
In fact, in the presence of a standard magnetic field (unidirectional) the two micro-magnets are attracted in the same direction.
So only one of the two magnets moves in the direction of the field, while the other remains locked.
Changing the operating pressure of the valve first requires the simultaneous unlocking of the two micro-magnets in the valve by a specific magnetic key. The rotor can then turn freely on its central axis.
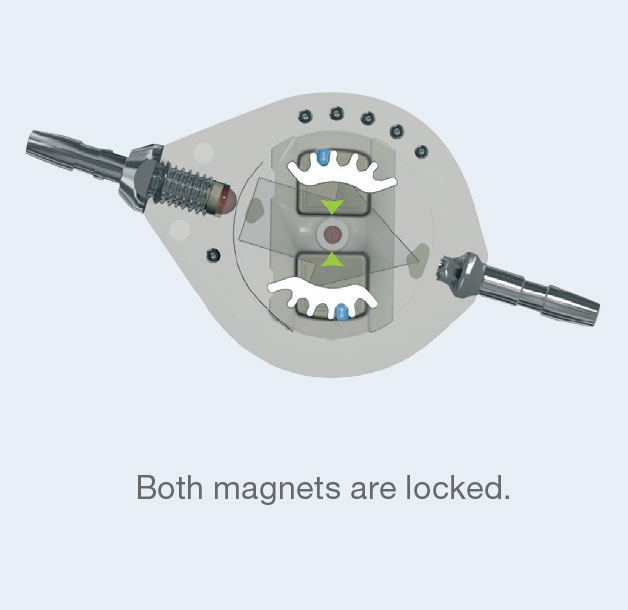
Valve subjected to a knock or to a magnetic field
with specific magnetic key

Direct pressure reading is obtained using the Adjustment Kit Compass: the Compass needle is aligned with the position of the magnetic rotor.
Whatever the medical device is, the final aim of the development process is to provide user a product with an acceptable risk/benefit ratio. On one hand we seek to lower the medical device related risk. On the other hand, we try to reach the highest levels of efficacy.
A common and important point of both elements of this ratio that drives the development of medical devices is the usability.
First, regarding the risk, a recent FDA recall study shown that 44% of medical device recalls are due to design problems, and user errors are often linked to the poor user experience.
On the other side, treating a patient with a medical device frequently requires a more complex sequence of actions than the “simple” delivery of a drug. Consequently the efficacy of a medical device is closely linked to the product usability.
Usability and user experience are the key ingredients to get an acceptable risk/benefit ratio.

At Sophysa, we believe that usability and user experience of medical devices should never be a brake to patient management. That is why in every development we do, we keep challenging the status quo to provide you a unique user experience enabling you to be totally dedicated to your patient.
Over the last 40 years, in accordance with our vision, we put all our effort in developing ground-breaking solutions improving user experience for a better patient management in the fields of CSF Management and Neuro ICU.
Here are some concrete and well-known example of this dedication to usability :

Sophysa is a French industry pioneer specialized in CSF Management, and Neuro Monitoring for Neuro ICU.
Over the last 40 years, in accordance to our commitment, we never stopped challenging status quo to improve patient management in improving user experience. Each time we develop a product, our passion for innovation, high technology and quality is serving our vision.

Over the last 40 years, our development has been driven by innovation, in particular through
Research and Development of unique and revolutionary solution.
The filing of dozens of patents for brands and mechanisms;
A brand new 2800 m2 manufacturing plant located with a 400 m2 clean room class 10,000 (ISO 7).
 PASSION FOR HIGH TECHNOLOGY
PASSION FOR HIGH TECHNOLOGYThe implantation of Sophysa production site in Besançon, capital of watchmaking and high precision is no coincid ! Sophysa has indeed always been driven by the ambition to develop its technological know-how, in areas as diverse as: precision thermoplastic injection molding silicone, treatment thermochemical carbon, encapsulation, the assembly of micro-sensors in silicon, miniaturization, ...
It first relied on the involvement and the unique skills of our collaborators, whose proven expertise allows them to make and finalize some precision parts manually, according to technics and methods developed and cultivated internally. This is the «made in France» quality that we proudly exports worldwide.

ICP Monitoring is a key element for patient management with acute brain damages. Over the last decades, the ICP monitoring has evolved from the simple average value calculated every hour to the analysis of the signal shape, its evolution in the time and the calculation of new indicators, all integrated in a multimodal monitoring.
Face to these new expectations, and in accordance with our commitment to develop ground-breaking solutions to improve user experience, our new ICP monitoring system Pressio®2 has been designed to bring:
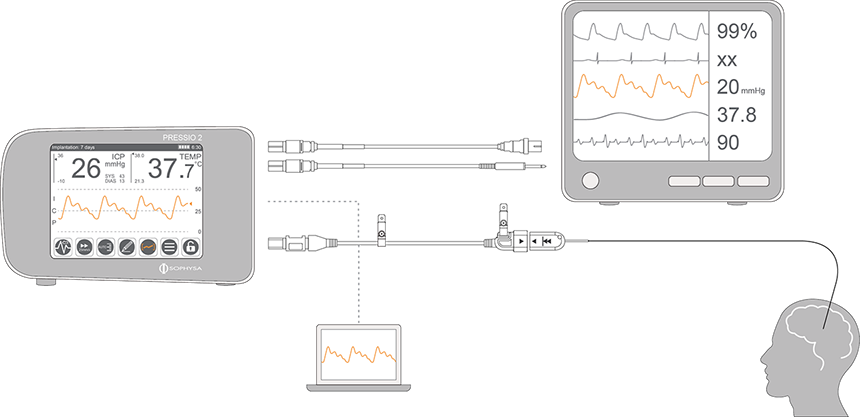
The Pressio® 2 system offers the possibility to transfer data of intracranial pressure and temperature monitoring either to a patient monitor or to a computer for research purposes, depending on clinicians’ needs.
For each type of transfer, different accessories are needed

Sophysa is a French industry pioneer specialized in CSF Management, and Neuro Monitoring for Neuro ICU.
Over the last 40 years, in accordance to our commitment, we never stopped challenging status quo to improve patient management in improving user experience. Each time we develop a product, our passion for innovation, high technology and quality is serving our vision.

Over the last 40 years, our development has been driven by innovation, in particular through
Research and Development of unique and revolutionary solution.
The filing of dozens of patents for brands and mechanisms;
A brand new 2800 m2 manufacturing plant located with a 400 m2 clean room class 10,000 (ISO 7).
 PASSION FOR HIGH TECHNOLOGY
PASSION FOR HIGH TECHNOLOGYThe implantation of Sophysa production site in Besançon, capital of watchmaking and high precision is no coincid ! Sophysa has indeed always been driven by the ambition to develop its technological know-how, in areas as diverse as: precision thermoplastic injection molding silicone, treatment thermochemical carbon, encapsulation, the assembly of micro-sensors in silicon, miniaturization, ...

It first relied on the involvement and the unique skills of our collaborators, whose proven expertise allows them to make and finalize some precision parts manually, according to technics and methods developed and cultivated internally. This is the «made in France» quality that we proudly exports worldwide.

Whatever the medical device is, the final aim of the development process is to provide user a product with an acceptable risk/benefit ratio. On one hand we seek to lower the medical device related risk. On the other hand, we try to reach the highest levels of efficacy.
A common and important point of both elements of this ratio that drives the development of medical devices is the usability.
First, regarding the risk, a recent FDA recall study shown that 44% of medical device recalls are due to design problems, and user errors are often linked to the poor user experience.
On the other side, treating a patient with a medical device frequently requires a more complex sequence of actions than the “simple” delivery of a drug. Consequently the efficacy of a medical device is closely linked to the product usability.
Usability and user experience are the key ingredients to get an acceptable risk/benefit ratio.

At Sophysa, we believe that usability and user experience of medical devices should never be a brake to patient management. That is why in every development we do, we keep challenging the status quo to provide you a unique user experience enabling you to be totally dedicated to your patient.
Over the last 40 years, in accordance with our vision, we put all our effort in developing ground-breaking solutions improving user experience for a better patient management in the fields of CSF Management and Neuro ICU.
Here are some concrete and well-known example of this dedication to usability :

ICP Monitoring is a key element for patient management with acute brain damages. Over the last decades, the ICP monitoring has evolved from the simple average value calculated every hour to the analysis of the signal shape, its evolution in the time and the calculation of new indicators, all integrated in a multimodal monitoring.
Face to these new expectations, and in accordance with our commitment to develop ground-breaking solutions to improve user experience, our new ICP monitoring system Pressio®2 has been designed to bring:

The Pressio® 2 system offers the possibility to transfer data of intracranial pressure and temperature monitoring either to a patient monitor or to a computer for research purposes, depending on clinicians’ needs.
For each type of transfer, different accessories are needed

The adeor nxt™ line of bipolar forceps also includes non-stick suction as well as irrigation forceps which combine our premium bipolar forceps technology with suction or irrigation capability.

Another advantage of our forceps is gained through the polishing process of the silver tips. This further increases the non-stick effect and also facilitates the cleaning and reprocessing procedures.

For the production of our nxt™ slimline bipolar cable, we use a specially developed manufacturing process. The Santropene™ components are directly sprayed onto the cable, resulting in an ultra-slim connector profile. A further benefit of this manufacturing technology is the guaranteed tightness of the cable, thereby ensuring a superior durability and long lifetime.
The nxt™ slimline bipolar cables offer a perfect ergonomical addition to every bipolar forceps on the market. The ultra-slim connector profile leads to a seamless extension of the forceps, without restricting the surgeon. Angled bipolar cables become redundant.


Another advantage of our forceps is gained through the polishing process of the silver tips. This further increases the non-stick effect and also facilitates the cleaning and reprocessing procedures.

The adeor nxt™ line of bipolar forceps also includes non-stick suction as well as irrigation forceps which combine our premium bipolar forceps technology with suction or irrigation capability.

For the production of our nxt™ slimline bipolar cable, we use a specially developed manufacturing process. The Santropene™ components are directly sprayed onto the cable, resulting in an ultra-slim connector profile. A further benefit of this manufacturing technology is the guaranteed tightness of the cable, thereby ensuring a superior durability and long lifetime.
The nxt™ slimline bipolar cables offer a perfect ergonomical addition to every bipolar forceps on the market. The ultra-slim connector profile leads to a seamless extension of the forceps, without restricting the surgeon. Angled bipolar cables become redundant.